Ice Table Chemistry Definition . an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. The ice table consists of several parts. ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction.
from solvedlib.com
An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. The ice table consists of several parts. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction.
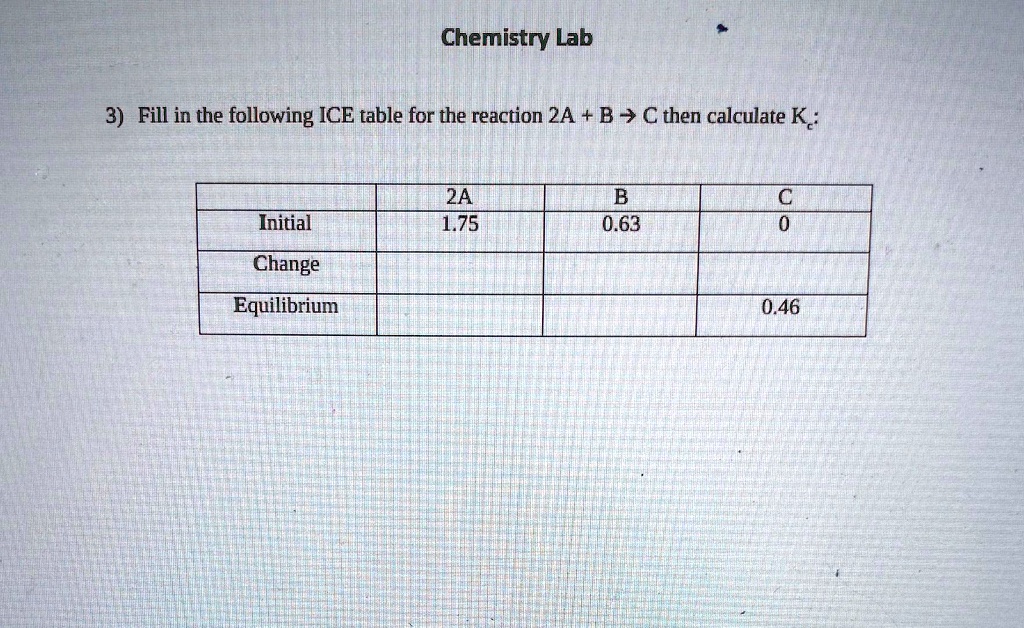
Chemistry Lab3) Fill in the following ICE table for t… SolvedLib
Ice Table Chemistry Definition ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. The ice table consists of several parts. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction.
From www.youtube.com
Extent of Reaction (ξ) ICE Tables in Physical Chemistry YouTube Ice Table Chemistry Definition An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze. Ice Table Chemistry Definition.
From www.showme.com
Ice table intro Science, Chemistry, Equilibrium ShowMe Ice Table Chemistry Definition ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. Ice. Ice Table Chemistry Definition.
From studyhypnotised.z21.web.core.windows.net
When Do You Use Ice Tables Ice Table Chemistry Definition The ice table consists of several parts. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. ice tables are a tool for organizing what is known and unknown in a. Ice Table Chemistry Definition.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing Ice Table Chemistry Definition an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations. Ice Table Chemistry Definition.
From www.youtube.com
Chemical Equilibrium Ice Table Equilibrium Constant Expression Ice Table Chemistry Definition ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. The ice table consists of several parts. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. Ice tables (initial, change, equilibrium) are a systematic method to. Ice Table Chemistry Definition.
From www.youtube.com
ICE Tables and Quadratic Formula, ICE Tables and Small Equilibrium Ice Table Chemistry Definition an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. The ice table consists of several parts. An ice (initial, change, equilibrium) table is simple matrix formalism that. Ice Table Chemistry Definition.
From exoktrtld.blob.core.windows.net
Ice Table For Diprotic Acid at Steven Mix blog Ice Table Chemistry Definition ice tables automatically set up and organize the variables and constants needed when calculating the unknown. ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the.. Ice Table Chemistry Definition.
From www.youtube.com
15.3 Equilibrium Calculations Using ICE Charts (aka ICE Tables Ice Table Chemistry Definition an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. Ice tables (initial,. Ice Table Chemistry Definition.
From solvedlib.com
Chemistry Lab3) Fill in the following ICE table for t… SolvedLib Ice Table Chemistry Definition Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. an ice table (acronym for. Ice Table Chemistry Definition.
From dxoniepxb.blob.core.windows.net
Ice Table In Chemistry at Terry Cordell blog Ice Table Chemistry Definition ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze. Ice Table Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Ice Table Chemistry Definition The ice table consists of several parts. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. ice tables are a tool for organizing what. Ice Table Chemistry Definition.
From www.youtube.com
ICE Tables in Chemistry Finding pH Using ICE Tables YouTube Ice Table Chemistry Definition an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. ice. Ice Table Chemistry Definition.
From dxoniepxb.blob.core.windows.net
Ice Table In Chemistry at Terry Cordell blog Ice Table Chemistry Definition ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. ice tables automatically set up and organize the variables and constants needed when calculating the unknown.. Ice Table Chemistry Definition.
From studyhypnotised.z21.web.core.windows.net
When Do You Use Ice Tables Ice Table Chemistry Definition The ice table consists of several parts. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible.. Ice Table Chemistry Definition.
From www.youtube.com
ICE Tables and Quadratic Formula, ICE Tables and Small Equilibrium Ice Table Chemistry Definition an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the. The ice table consists of several parts. an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. ice tables are a tool for. Ice Table Chemistry Definition.
From chemguru.sg
determineequilibriumconstanticetable Ice Table Chemistry Definition An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. an ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. an ice table. Ice Table Chemistry Definition.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership Ice Table Chemistry Definition The ice table consists of several parts. An ice (initial, change, equilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. an ice table is used to organize the information for the initial, change, and equilibrium concentrations of the reaction. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants. Ice Table Chemistry Definition.
From www.youtube.com
14.5 ICE Table Examples and Approximations YouTube Ice Table Chemistry Definition ice tables are a tool for organizing what is known and unknown in a stoichiometry or equilibrium problem. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an ice table, also known as an initial, change, and equilibrium table, is a tool used in chemistry to organize and analyze the.. Ice Table Chemistry Definition.